Oncology Overview
Cancer Cells Evolve, So Does Our Science
- We are pursuing a highly promising pipeline of next generation oncology leads by creating discovery platforms around validated oncogenic pathways with clinical de-risking.
- Our platforms harbor next generation differentiated leads that specifically target the oncoproteins as the backbone program with further assets that allow for a deeper exploration of the target biology & indication space.
- Through this platform approach, Rhizen is looking to develop a derisked pipeline of novel oncology agents that can achieve better treatment outcomes and expand into novel indications & strategic combinations.
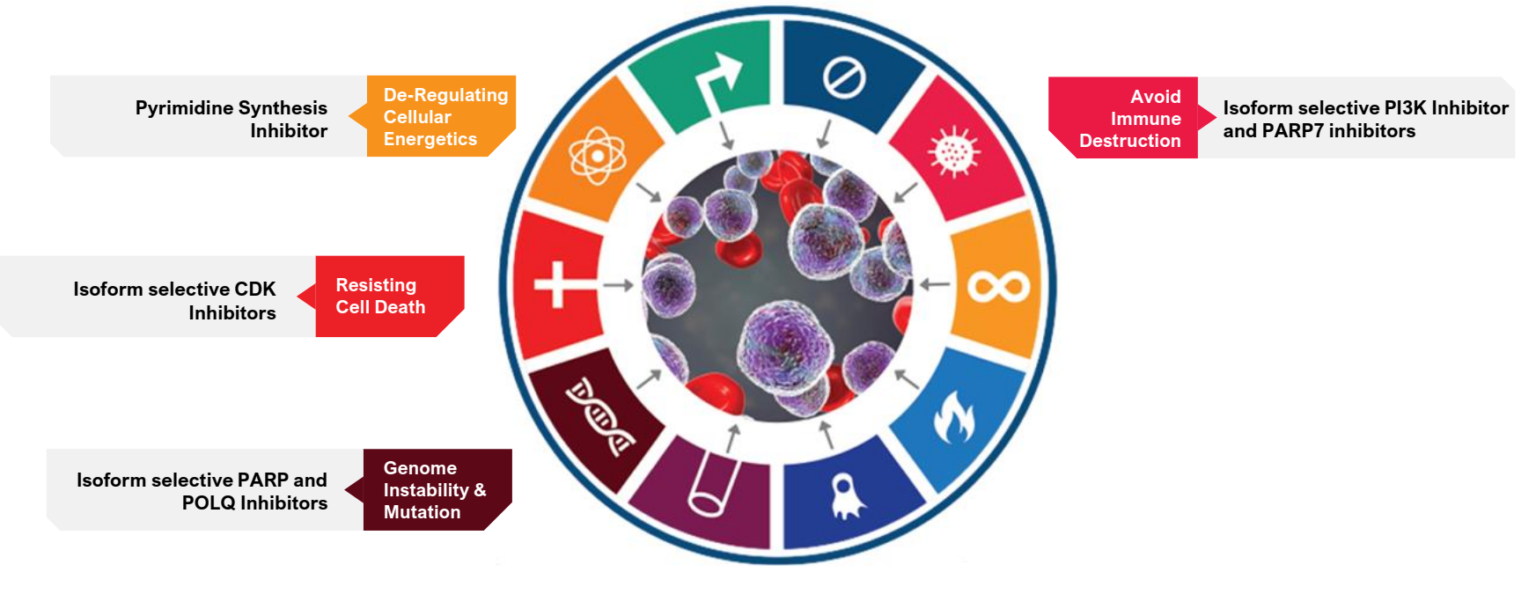
We employ a multi-pronged approach by modulating two or more ‘hallmark’ mechanisms simultaneously |
 |
This approach guides our choice of molecular targets and our pipeline of novel cancer medicines |
To know more about our assets targeting these multiple hallmarks of cancer, visit our
The Oncology Journey
Tracing Our Journey in Identifying and Developing Key Assets based on identified platforms to address multi-evasive biology
- Early Efforts
- Pioneering Research
- Global Outlook
- Expanding Horizons
- Regulatory Progress
- Regional Deals
- Future Steps
Early Efforts
Early programs in the isoform selective PI3K space hit safety issues; Rhizen sees opportunity for safer, differentiated molecules. Discovery approach is based on leveraging proven science to nurture beneficial clinical positioning
Pioneering Research
Development & optimization of leads in isoform selectivePI3K
Rhizen’s leads are specifically designed to limit the immune-related toxicity known and improvise the ability to combine with complementary assets.
Early success guided the research philosophy to create platforms to pursue and explore the full potential of associated biology
Global Outlook
Licensing deal with TG Therapeutics; First Program from platform initiation moves into the clinic for B cell lymphomas both as monotherapy and anti-CD20 combination
Expanding Horizons
Rhizen leverages existing drug discovery capabilities; Differentiated asset Tenalisib starts pre-clinical journey, multiple assets followed to clinic
Regulatory Progress
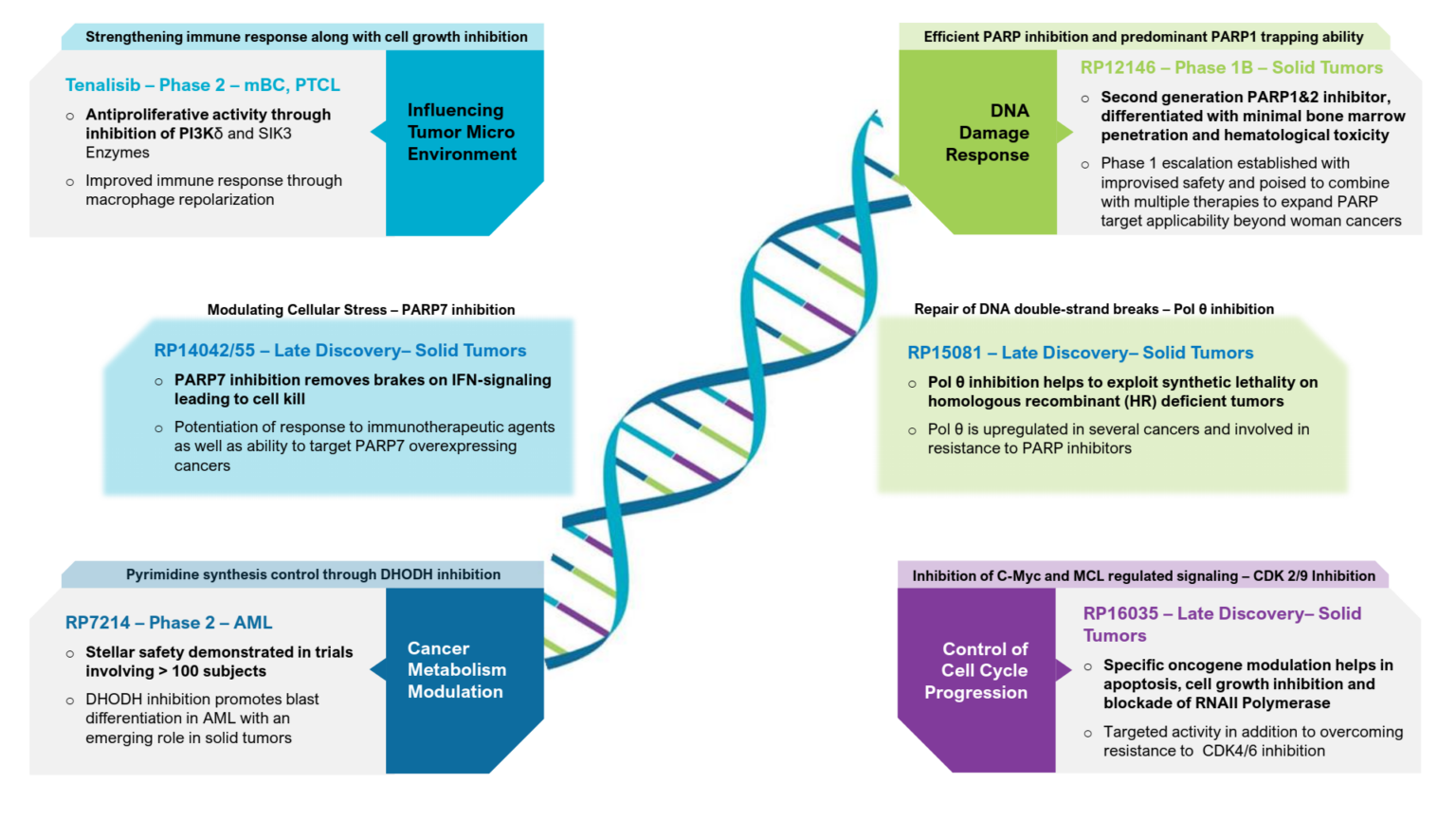
Tenalisib - potential best in class PI3Kδ/γ Inhibitor with SIK3 Modulation is positioned, with established rationale, for TCL, Solid Tumors, MF and AML in combinations
Inflammation programs - oral and inhaled - have proven the safety of leads selected in global clinical trials
Oncology platforms expanded substantially exploring pathways in - Cancer Metabolism, DNA Damage Response, Cyclin Dependent Kinases - aiming at multiple best or first in class oncology assets.
Regional Deals
Rhizen signs a deal with Curon Biopharma for Greater China rights of Tenalisib. Clinical development in China initiated
Future Steps
Multi-Pronged Science
Programs along with combinations are pursued to mitigate risks associated with targeted drug development in Oncology
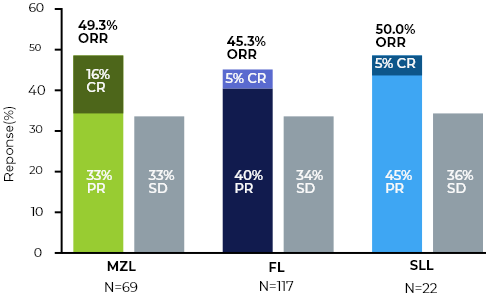
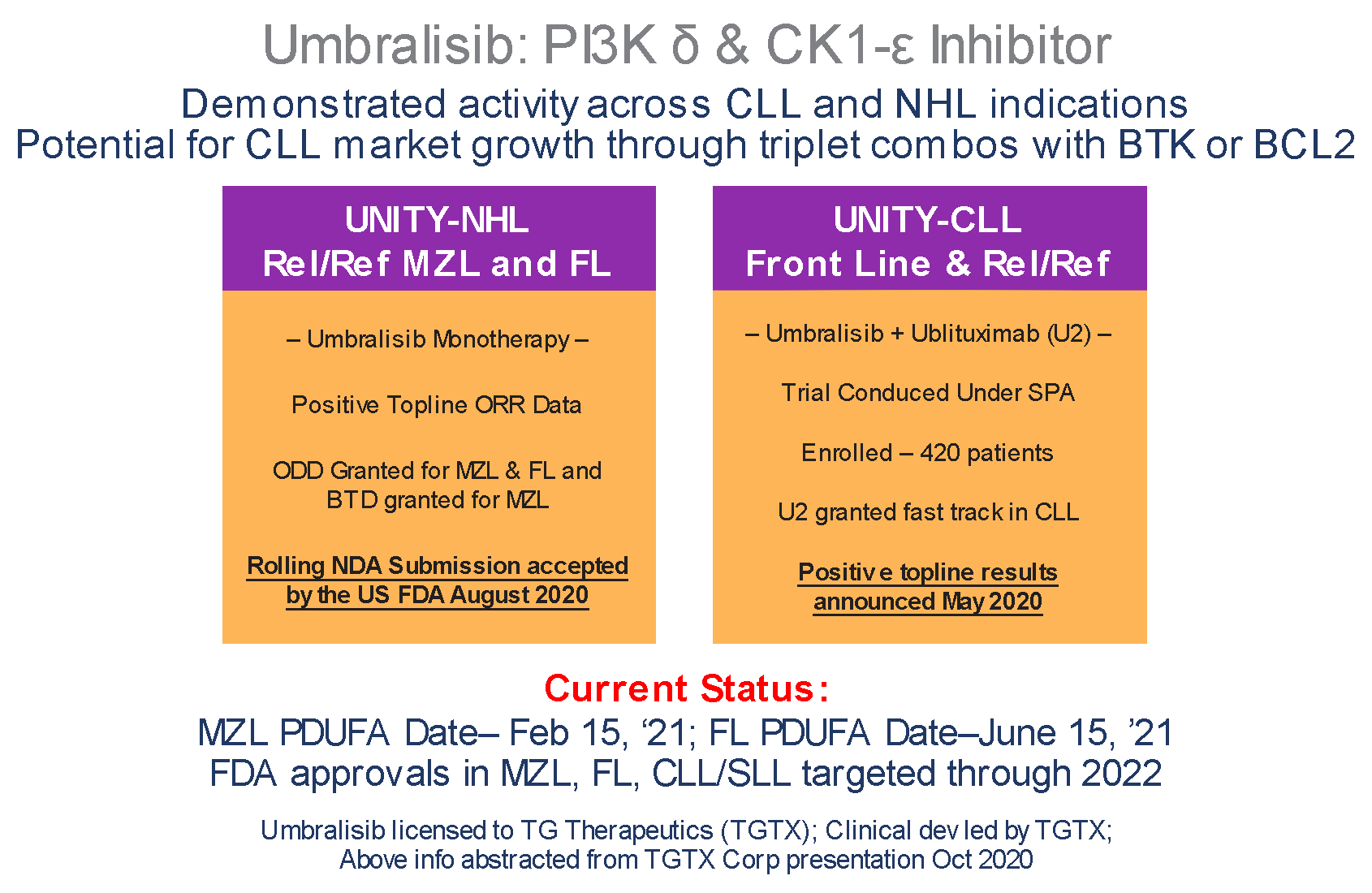
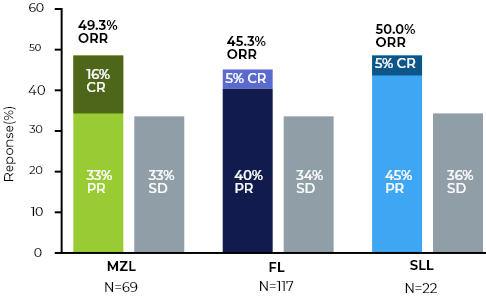
- Durable single agent responses across R/R iNHLs
- No MZL Complete Responses have progressed
Manageable safety profile - Low incidence of immune mediated toxicities and AE related discontinuations
UNITY-CLL: Front Line & R/R CLL Umbralisib + Ublituximab (U2) combo improved PFS
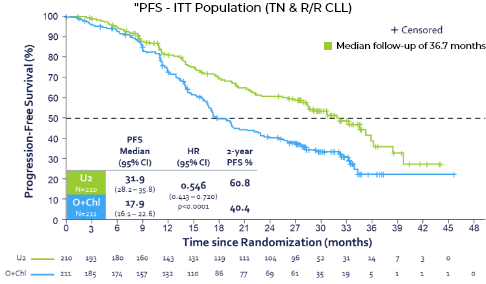
- Trial conducted under SPA enrolled1L & R/R CLL pts
- N=421
Control: Obinutuzumab + Chlorambucil - Met the primary endpoint of improved PFS (p<.0001>
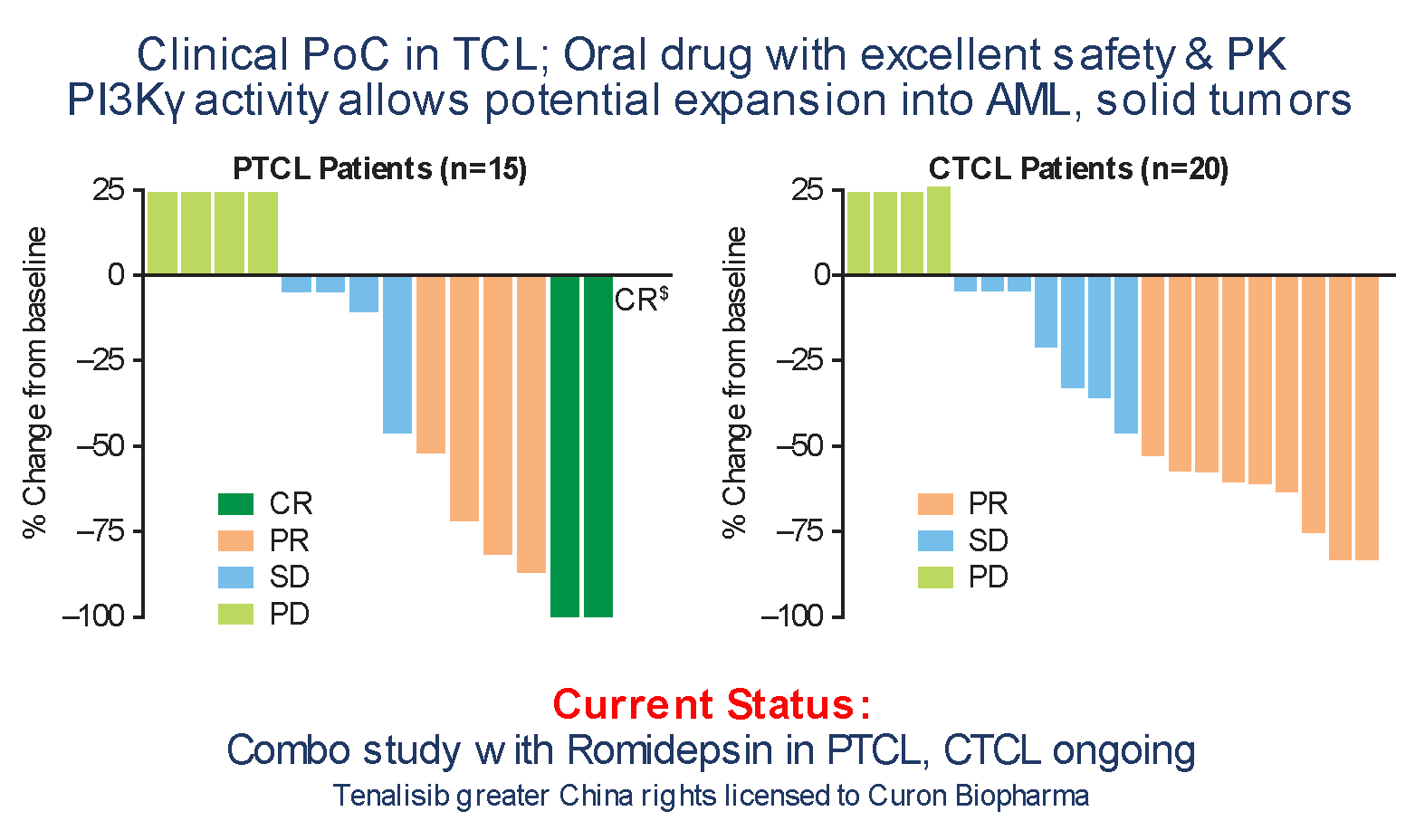
Tenalisib: Dual PI3K δ and γ, SIK3 Inhibitor
PTCL Patients(n=15)
CTCL Patients(n=20)
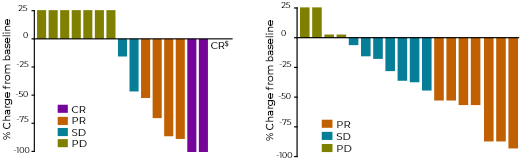
$- Non-measurable disease; positive bone marrow became negative (CR).
Cancers(Basel). 2020 Aug. Huen.et.al: Tena Mono in R/R TCL
- Robust single agent activity in PTCL/CTCL (mITT)
- R/R PTCL – ORR - 47%
mDoR - 6.53 months(0.97 - 21.0) - R/R CTCL – ORR - 45%
mDoR - 3.8 months(2.3 - 12.8)
- R/R PTCL – ORR - 47%
- Safe and well tolerated
- No colitis; Very low neutropenia
- Related Grade ≥ 3 AEs - transaminitis (AST/ALT) (21%) & rash (5%)
Phase I/II Study of Tenalisib with Romidepsin in R/R TCL
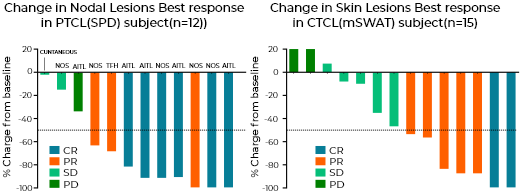
- Overall efficacy responses in both PTCL & CTCL looks encouraging (mITT)*
- R/R PTCL – ORR - 75% (6 CRs, 3 PR, 2 SDs); mDoR - 4.67(2.1 - NR)
- R/R PTCL – ORR - 75% (6 CRs, 3 PR, 2 SDs); mDoR - 4.67(2.1 - NR)
- No unexpected AEs or increased frequency of existing AEs for individual agents were observed
- Study enrollment has been completed; patient evaluation is ongoing
Our PI3K Assets
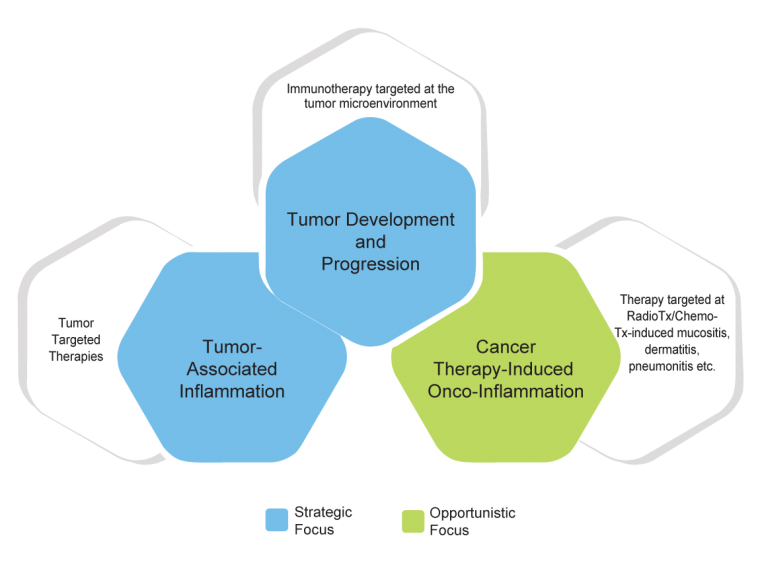
Onco Inflammation
Cancer and Inflammatory Processes and Targets Are Often Linked, so is Our Opportunistic/Licensing Focus
To know more about our Onco-Inflammation licensing opportunities, visit our Pipeline Page
Clinical Trials
Program |
ID |
Title |
Phase |
Status |
Conditions |
Locations |
|---|---|---|---|---|---|---|
| Selective Pi3K Inhibitor | Efficacy and Safety of Tenalisib (RP6530), a PI3K δ/γ and SIK3 Inhibitor, in patients with Locally Advanced or Metastatic Breast Cancer |
Phase 2 |
Active |
Locally Advanced or Metastatic Breast Cancer |
Georgia |
|
Safety and Efficacy of Tenalisib (RP6530) in Combination With Romidepsin in Patients With Relapsed/Refractory T-cell Lymphoma |
Phase 1/2 |
Recruitment Completed |
T-cell Lymphoma |
USA |
||
Compassionate Use Study of Tenalisib (RP6530) |
Phase 1/2 |
Enrolling by Invitation |
Hematological Malignancies |
USA, Georgia, Poland |
||
| DHODH Inhibitor | A Single and Multiple Ascending and Food Effect Study of RP7214, a DHODH Inhibitor in Healthy Adult Subjects |
Phase 1 |
Completed |
Healthy Volunteers |
USA |
|
Study to Evaluate the Efficacy and Safety of Oral RP7214, a DHODH Inhibitor, in Patients With Symptomatic Mild SARS-CoV-2 Infection |
Phase 2 |
Completed |
Mild SARS-CoV-2 Infection |
India |
||
PARP Inhibitor |
Safety, Pharmacokinetics and Anti-tumor Activity of RP12146, a Poly (ADP-ribose) Polymerase (PARP) inhibitor, in Patients with Locally Advanced or Metastatic Solid Tumors |
Phase 1 |
Recruiting |
Advanced or Metastatic Solid Tumors |
Poland and Czech-Republic |
Scientific Advisory Board
Dr. James E. Sanders, DVM, PhD, DABT
as a staff pathologist in the U.S. Army at Walter Reed Army Institute of Research and from 1981 to 1987 as a Veterinary Pathologist at Merck, Sharp and Dohme. Joined Rhône-Poulenc Rorer as Director of Toxicology in 1987 and continued through mergers (Aventis, Sanofi Aventis) until 2005. Here, as Global Head of Toxicology, had worldwide responsibilities for designing and implementing the nonclinical toxicology and safety pharmacology testing of all drug candidates developed by the company. Was responsible for the preparation of the nonclinical sections for all INDs, NDAs, CTXs, and MAAs and for the communications with government Health Authorities globally. From 2005 to 2007 was Senior Director at Johnson & Johnson, directing development of pharmaceuticals from early development through NDA and MAA approval including government Health Authority interface. In these positions, has represented his company externally in forums and working parties such as the CPMP’s SWP, DIA, ILSI, Toxicology Forum, ICH and PhRMA. Attended Ohio State University gaining a DVM, an MS in Pharmacology and a PhD in toxicology. Is certified by the American Board of Toxicology and is a member of the SOT, and the Society of Toxicological Pathologists.…Read Less
Dr. T. S. Ganesan M.D, Ph.D
He completed his training in Medical Oncology at St. Bartholomew’s Hospital, London. His doctorate was on Philadelphia chromosome positive leukemias that was awarded by the University of London. He was subsequently appointed as Consultant Medical Oncologist at Churchill Hospital, Oxford and established a laboratory as Clinical Scientist at the Weatherall Institute of Molecular Medicine, Oxford. After 15 years at Oxford he was appointed as Chairman, Cancer Institute and Institute of Molecular Medicine at Amrita Institute of Medical Sciences, Cochin, India towards the end of 2005. His research interests have been on cancer genetics and signal transduction, in addition to clinical trials. The research in UK was supported initially by the Imperial Cancer Research Fund and subsequently by Cancer Research UK. In India, the research is supported by Department of Biotechnology and Indian Council of Medical Research. He has been awarded the Clinical Excellence award in UK during his tenure there. He has over 100 papers to his credit in international journals. …Read Less
Dr. Dhanapalan Nagarathnam Ph.D
cancer indications, diabetes, autoimmune disorders, HCV, anti-bacterial, inflammatory bowel disease (IBD), etc. Prior to this, Dr. Nagarathnam was the Director of Medicinal Chemistry at Pharmasset, Princeton, NJ, wherein he directed the HCV drug discovery projects and played a key role in selecting three liver-targeted clinical candidates and the most advanced candidate is in Phase-III human trials, and the company was later acquired by Gilead in 2012. Dr. Nagarathnam also worked as Principal Research Scientist-II at Bayer HealthCare, West Haven, CT, in multiple oncology, diabetes, obesity and osteoporosis projects and Synaptic Pharmaceutical Corporation (currently Lundbeck), Paramus, NJ on multiple human GPCR-targeted projects targeting BPH, pain, obesity, etc. In the Synaptic/Merck collaboration project he contributed to nomination of two alpha-1a adrenoceptor selective candidates for the treatment of Benign Prostatic Hyperplasia (BPH).Dr. Nagarathnam has over 25 years of experience in pharmaceutical industry and this includes leadership role in project management, medicinal chemistry, combinatorial chemistry, high-throughput synthesis, lead generation, lead optimization, process research, production, and business development. Dr. Nagarathnam obtained his Ph. D. in Organic Chemistry from the University of Madras, India and conducted postdoctoral research in Medicinal Chemistry at University of California, Purdue University, and University of Illinois and process research at Malti-Chem Research Center. Overall, Nagarathnam is an inventor on over 50 issued patents/patent applications, and an author of over 75 peer-reviewed publications in leading journals and conference presentations. …Read Less