The Rhizen Journey!
Our Journey to be a Leading Discovery & Development Company Focusing on Differentiated Products Through Cutting-edge Science and Strategic Partnerships.
.

- 2008-09
- 2010-11
- 2012-13
- 2014-15
- 2016-17
- 2018-19
- 2020-21
- 2022-25
First Steps
Established as discovery and development company
Progressed focusing on Oncology and Inflammation – globally competing science
Setting the Roadmap
Development of an early stage pipeline and assets.
Collaborations for translational medicines
Efforts Pay Off!
Partnership with TG Therapeutics – IND ready TGR1202
Joint development of TGR1202 through Phase 1
Nomination of further leads for clinical development

Branching Out
Licensing of TGR1202 for global development to TG Therapeutics
Licensing deal with Novartis AG for RP6557 for global development of non-oncology indications
Establishment of clinical development function and execution of global trials.
Key Asset Primed for Success
Maturation of our alliances with TG and Umbralisib (TGR1202) poised for success with Phase 3 global trial initiated
RP6530 granted Orphan, fast-track status by US FDA for treating T Cell Lymphomas
Aspirational Steps
Rapid expansion of clinical pipeline
Articulation of our ambition to pursue late-stage development
Initiation of Tenalisib Phase POC Studies
Focus on Oncology & Inflammation
Tenalisib demonstrates promising clinical POC and class leading safety across monotherapy and combination settings in PTCL & Solid Tumors. Significant improvement in DCR in 3L HR+, HER2-, mBC paves way for future development
RP7214, Cancer Metabolism targeted lead enters POC clinical trial in AML
RP12146, Second Generation PARP1 & 2 inhibitor progresses to Phase 1/1B across solid tumors
Inflammation assets are positioned for topical applications through inhalation progressing to clinical development in respiratory indications
Drug discovery efforts extended to Precision Oncology targeting Pol θ, PARP7 and CDK2/9 enzymes
Our Target
Full scientific and collaborative evolution for value creation
Our Leadership
Proven and seasoned senior leadership team with expertise spanning the entire discovery-development-commercialization cycle.
The Rhizen Board

Mr. Pranav Amin
Mr. Pranav Amin is the Managing Director of Alembic Pharmaceuticals Ltd. and heads the International Business Unit of the Organization. He joined the organization in 2007 as a Director and was elevated to the position of Joint Managing Director in 2015. In April 2016, he took charge as a Managing Director. A graduate in Economics/Industrial Management from the Carnegie Mellon University in Pittsburgh, USA and MBA in International Management from Thunderbird, Arizona State, USA, he is a great people leader and leads his enterprise through involvement, empowerment, and autonomy. He is also a Trustee of the Bhailal Amin General Hospital and the Uday Education Society, a trust which runs four schools in Vadodara.

Dr. Swaroop Vakkalanka
Swaroop Vakkalanka is a renowned pharma R&D professional with rich and rewarding experience in research and strategy management. He led diversified scientific teams successfully from drug design to clinical development. He played a pivotal role in bringing 18 NCEs into clinical development right from concept. He served in R&D divisions of leading Indian pharmaceutical companies before founding Rhizen Pharmaceuticals SA. To his credit he led the R&D teams for several out-licensing deals in the areas of Oncology & Inflammation, Pain and Metabolic disorders with cumulative deal value of > $ 1500 Million. Swaroop obtained his Ph.D in Pharmaceutical Sciences from Andhra University, India.

Swaroop Vakkalanka, PhD
Founder & CEO
Multi-scientific team leader in drug discovery and development.
Proven
track record in best enterprise value creation through successful implementation of R&D strategies
Swaroop Vakkalanka, PhD
Founder & CEO

Ajit Nair, PhD
Chief Development Officer
23+ years of experience in clinical development and medical affairs in general medicine and oncology dev across different phases of development.
Leading the team that develops and executes the clinical strategy.
Ajit Nair, PhD
Chief Development Officer
What We Do
Corporate Structure
End to end capabilities to discover and develop novel NME's
Pipeline of differentiated assets with first/best-in-class potential
Discovery platforms structured with multiple assets to explore true potential of selected pathways
Robust development capabilities integrated to help swift clinical development
Track record of successful collaborations with global partners
Flexible, out-licensing for developed markets; retain regional rights

Niche-focused Swiss HQ Biotech with global operations

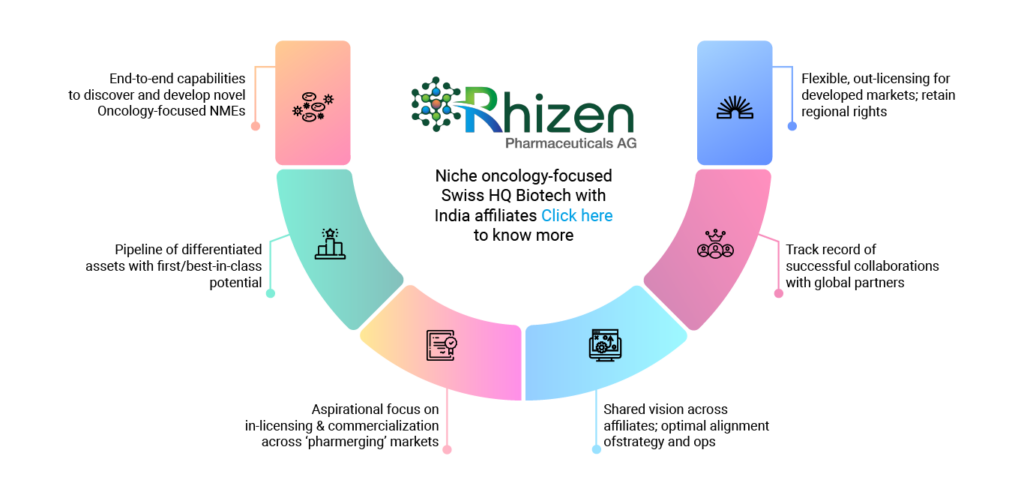

End-to-end capabilities
to discover and
develop novel
Oncology-focused NMEs

Flexible, out-licensing for developed markets; retain regional rights

Pipeline of
differentiated
assets with
first/best-in-class potential
Track record of successful
collaborations
with global partners

Aspirational focus on in-licensing & commercialization across ‘pharmerging’ markets
Shared vision
across affiliates;
optimal
alignment of
strategy and ops

Key Strengths
Deep pipeline of assets that addresses multiple indications with deeper focus
Proven expertise in discovery, preclinical, CMC, clinical development and regulatory affairs


Ability to execute development across geographies & regulatory agencies with relevant designations (orphan, breakthrough, fast-track etc)
Access to and oversight of 'at-scale' manufacturing operations and commercial footprint

Our Network
Our R&D operations and collaborations extend across the globe
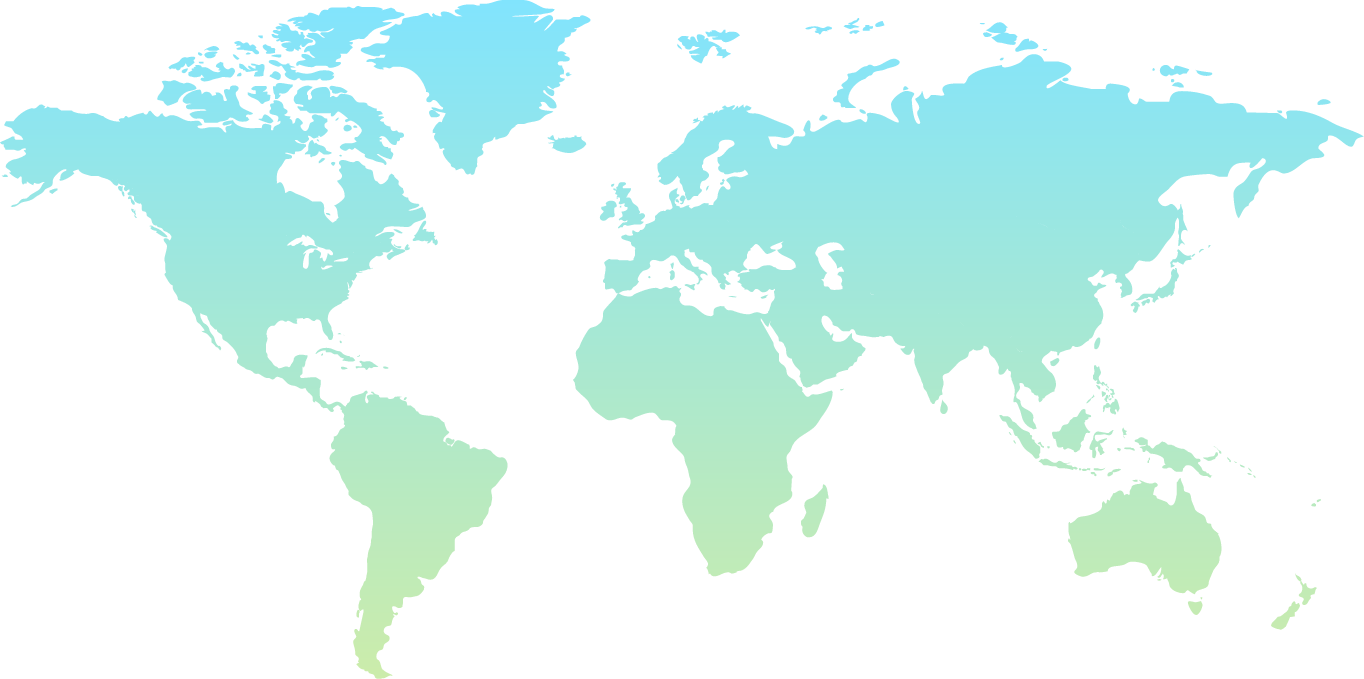
USA – Multiple Locations
Ongoing Clinical Trial
Phase 1/2 – Tenalisib in Hematological Malignancies
USA – Multiple Locations
Ongoing Clinical Trial
Phase 1/2 – Tenalisib in Hematological Malignancies
USA – New York
Licensing Partner of TGR 1202
TG Therapeutics
Poland
Ongoing Clinical Trial
Phase 1/2 – Tenalisib in Hematological Malignancies
Poland
Phase 1 – RP 7214 in Advanced or Metastatic Solid Tumors
Czech Republic
Active Clinical Trial
Phase 1 – RP 7214 in Advanced or Metastatic Solid Tumors
Switzerland
Rhizen HQ
Georgia
Ongoing Clinical Trial
Phase 1/2 – Tenalisib in Hematological Malignancies
Georgia
Shareholder/Partner Company
Alembic Pharmaceuticals
UAE - Dubai
Rhizen Branch
INDIA - BARODA
Shareholder/Partner Company
Alembic Pharmaceuticals
INDIA
Phase 1
RP 7214 in Mild SARS-CoV-2 Infection
India - Hyderabad
Affiliate Company
Incozen Therapeutics
China
Licensing Partner of RP 6530
Curon BioPharma
Clinical Trial Locations
Rhizen Offices
Partner Companies
Affiliate Companies
Explore opportunities to collaborate across the discovery-development-commercialization value chain.